A research team has developed a technology that dramatically enhances the stability of ultra-thin metal anodes with a thickness of just 20μm. Led by Professor Yu Jong-sung from the Department of Energy Science and Engineering at DGIST, the team proposed a new method using electrolyte additives to address the issues of lifetime and safety that have hindered the commercialization of lithium metal batteries. The work is published in the journal Advanced Energy Materials.
Lithium metal anodes (3,860 mAh g⁻¹) have over 10 times the capacity of widely used graphite anodes (372 mAh g⁻¹) and feature a low standard reduction potential, making them promising candidates for next-generation anode materials. However, during charge-discharge cycles, lithium tends to grow in dendritic forms, causing short circuits and thermal runaway, which leads to lifetime and safety issues. Moreover, due to volume expansion, the solid electrolyte interphase (SEI) repeatedly degrades and reforms, leading to rapid electrolyte depletion.
The use of ultra-thin lithium metal with a thickness below 50μm is essential, especially for the commercialization of lithium metal batteries. However, such issues become more severe as thickness reduces. Accordingly, both academia and industry have focused on SEI engineering to enhance the stability of lithium metal anodes, among which SEI formation strategies using electrolyte additives have emerged as a simple yet effective approach.
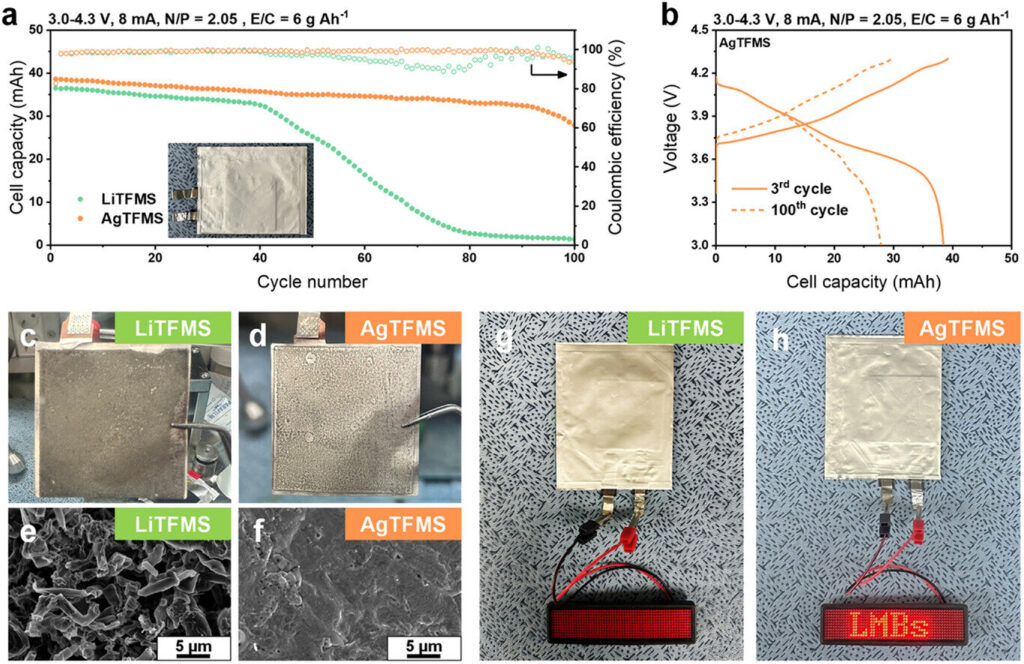
Previous studies have shown that lithium fluoride (LiF) contributes to the enhanced stability of lithium (Li) metal anodes due to its high mechanical strength. More recently, silver (Ag) has also been reported to promote uniform lithium deposition through an alloy reaction with Li. However, no research has yet explored a single additive capable of simultaneously forming both Ag and LiF.

To this end, Professor Yu’s team introduced silver trifluoromethanesulfonate (AgCF₃SO₃, or AgTFMS) as an electrolyte additive to address dendrite formation and poor cycle life. Through various surface analyses, the team confirmed that using an AgTFMS-containing electrolyte leads to the simultaneous formation of Ag and LiF on the lithium metal surface.
Based on this, they successfully enhanced the stability of ultra-thin (20μm) lithium metal anodes and experimentally verified that dendrite formation could be effectively suppressed and the battery life could be extended by more than seven times compared to the conventional system. Simultaneously, Professor Kang Jun-hee’s team at Pusan National University employed computational chemistry to analyze the interaction energy between Li and Ag, thereby elucidating the underlying mechanism for enhanced stability.
Professor Yu Jong-sung of DGIST stated, “This study focused on overcoming the limitations of ultra-thin lithium metal and significantly enhancing the stability of lithium metal batteries. By forming a high-performance SEI through a simple approach, we have developed a technology that improves both the lifetime and efficiency of lithium batteries.
“We expect that this advancement will accelerate the commercialization of lithium metal batteries as sustainable energy storage systems across various applications, including electric vehicles, unmanned aerial vehicles, and ships.”
More information:
Jong Hun Sung et al, Dynamic Cycling of Ultrathin Li Metal Anode via Electrode–Electrolyte Interphase Comprising Lithiophilic Ag and Abundant LiF under Carbonate‐Based Electrolyte, Advanced Energy Materials (2025). DOI: 10.1002/aenm.202500279
Citation:
Electrolyte additives enhance ultra-thin lithium metal anodes for longer-lasting batteries (2025, April 9)
retrieved 10 April 2025
from https://techxplore.com/news/2025-04-electrolyte-additives-ultra-thin-lithium.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.